Dietary Iron Oxidation State

Other possible oxidation states for iron includes.
Dietary iron oxidation state. The highest known. Four major classes of iron containing proteins exist in the mammalian sys tem. 5 4 3 and 2. Ferrum and atomic number 26.
The modern names reflect the oxidation. Ok so what are the common oxidation states for an atom of fe. There is also a compound feso 3 with the old name of iron ii sulphite. Iron is an essential bioelement for most forms of life from bacteria to mammals its importance lies in its ability to mediate electron transfer.
Here is a chart which shows the most common oxidation states for first row transition metals. In chemistry iron iii refers to the element iron in its 3 oxidation state in ionic compounds salts such an atom may occur as a separate cation positive ion denoted by fe 3. At low ph iron prefers to stay as fe 2. Since there are many exceptions to the formula it would be better just to memorize the oxidation states for the fourth period transition metals since they are more commonly used.
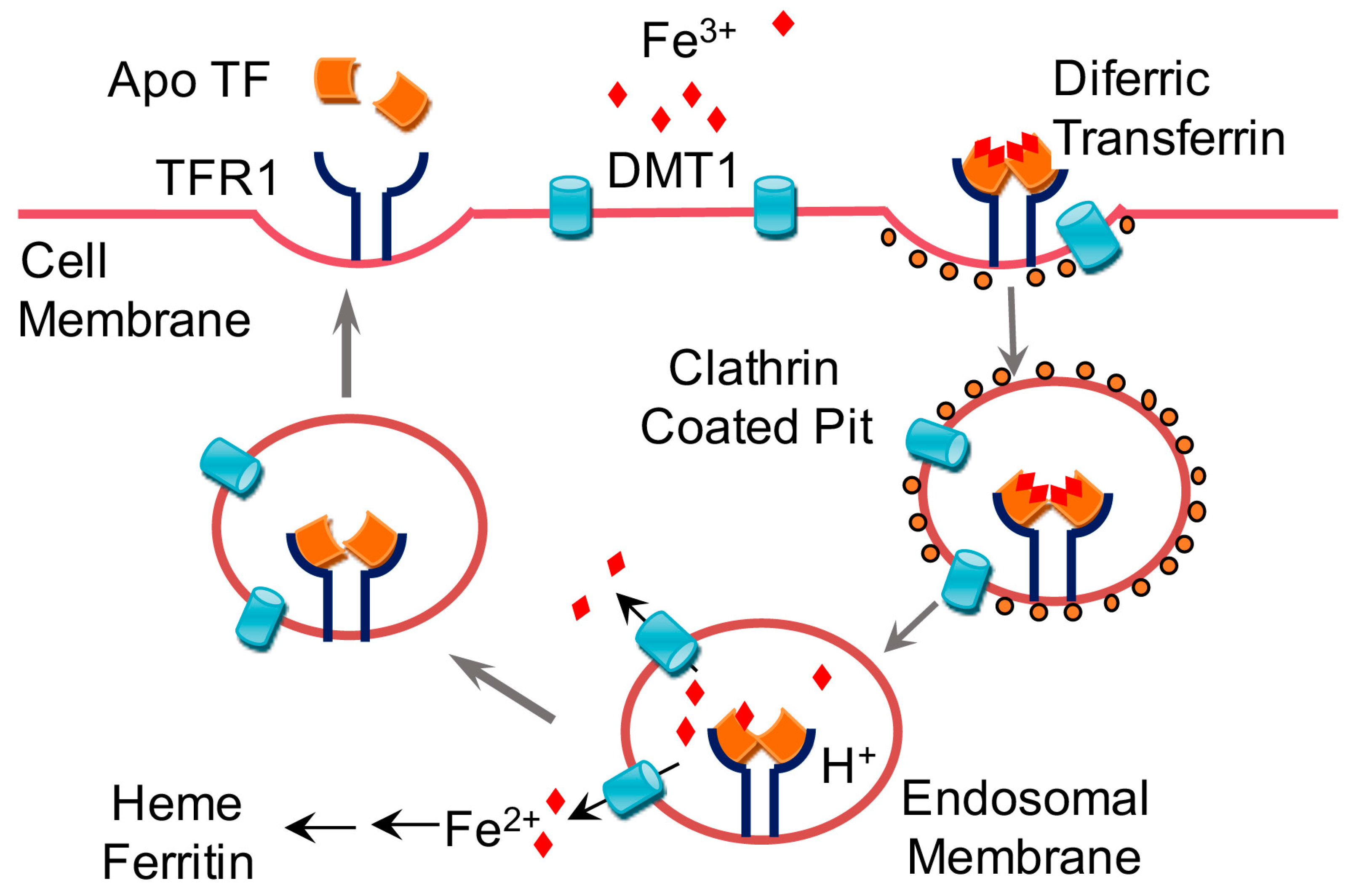
The ii and iii are the oxidation states of the iron in the two compounds. Heme iron found primarily in red meats is the most easily absorbed form. There are two major forms of dietary iron. However as ph increases it rapidly oxidizes to fe 3 to form fe oh 3 which with time precipitates as rust.
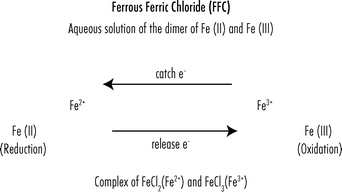
In the ferrous state iron acts as an electron donor while in the ferric state it acts as an acceptor thus iron plays a vital role in the catalysis of enzymatic reactions that involve electron transfer reduction and oxidation redox. The oxidation state of iron is dependent on both ph and redox potential. The oxidation state of an atom is not regarded as the real charge of the atom. It is a metal that belongs to the first transition series and group 8 of the periodic table.
Iron ˈ aɪ ər n is a chemical element with symbol fe from latin. In the case of iron the most common oxidation states is 2 3 there are also cool facts about iron that most don t know about. It is the fourth most common element in the. This can also be extended to the negative ion.
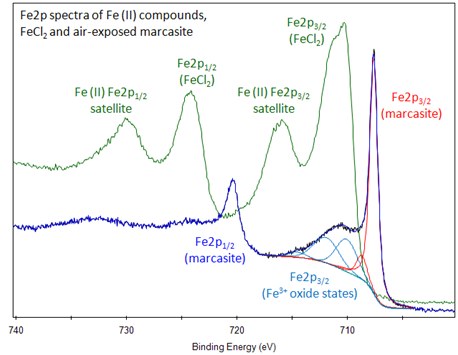
Learn more about the oxidation states here. That tells you that they contain fe 2 and fe 3 ions. 2 and 3 respectively. Iron containing heme proteins hemoglobin myoglobin cytochromes.
Likewise the oxidative state of iron is dependent on the redox potential e h of the environment. It is by mass the most common element on earth right in front of oxygen 32 1 and 30 1 respectively forming much of earth s outer and inner core. The adjective ferric or the prefix ferri is often used to specify such compounds as in ferric chloride for iron iii chloride fecl 3 the adjective ferrous is used instead for iron ii salts containing. Iron ii sulphate is feso 4.
The increased requirement for dietary iron for boys and girls in the growth spurt is 2 9 mg day and. The lowest known oxidation state is 4 for carbon in ch 4 methane. In a chemical reaction if there is an increase in oxidation state then it is known as oxidation whereas if there is a decrease in oxidation state it is known as reduction.