Dietary Supplement Health And Education Act Ppt

Because of the dietary supplement health and education act of 1994 the special rules that the fda uses state that the manufacturer is responsible for ensuring that a dietary supplement is safe before it is marketed and the fda is responsible for taking action against any unsafe dietary supplement product after it has reached the market.
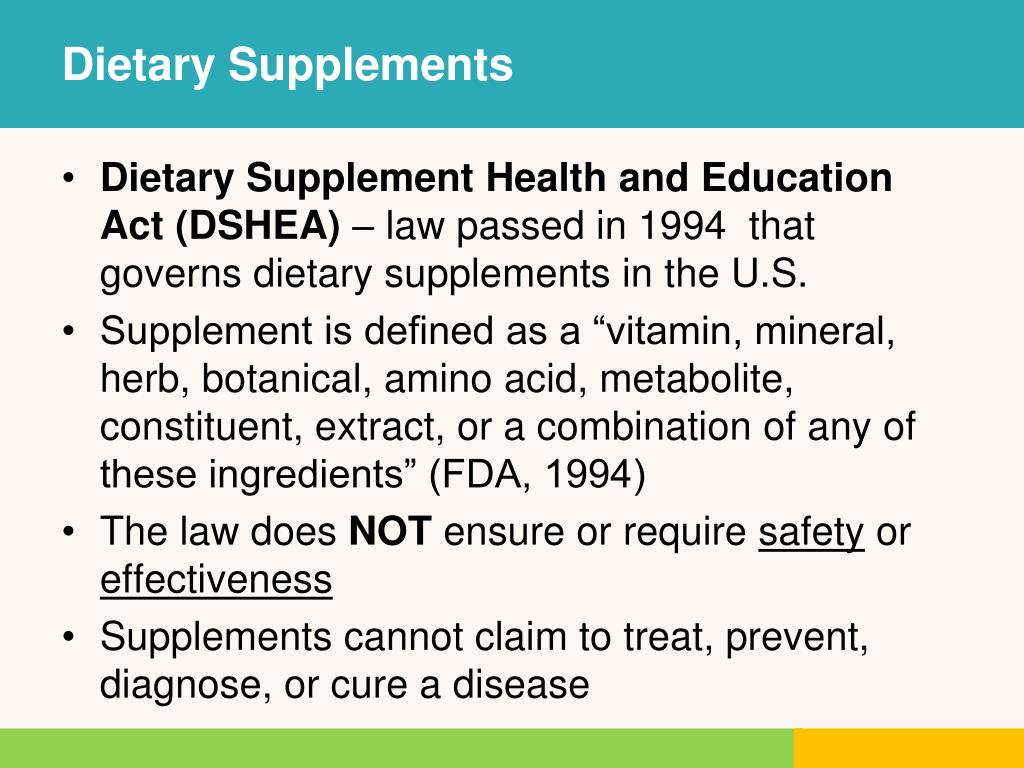
Dietary supplement health and education act ppt. Dietary supplement health and education act of 1994 the house of representatives adopted on octo ber 7 a revised version of s. Powerpoint ppt presentation free to view. 784 and the senate concurred in that amendment on october 9 sending new dietary supplement legislation to the president who is expected to sign it into law. Dietary supplement health and education act of 1994 definition of dietary supplement is a product other than tobacco that is intended to supplement the diet that bears or contains one or more of the following dietary ingredients.
This act may be cited as the dietary supplement health and education act of 1994. Campbell university last modified by. Labeling dietary supplements 1 in our post about the nutraceuticals industry dietary supplements as a category of nutraceuticals are subject to compliance with fda regulations under the dietary supplement health and education act of 1994 dshea. A vitamin a mineral an herb.
Dietary supplement health and education act of 1994 author. A vitamin a mineral an herb or other botanical an amino acid a dietary substance for use by man to supplement the diet by increasing the total daily intake or. This is the first major. Whenever in this act an amendment or repeal is expressed in terms of an amendment to or repeal of a section or other provision the reference shall be considered to be made to a section or other provision of the federal food drug and cosmetic act.
The dietary supplement health and education act of 1994 which spells out regulations regarding the manufacture and sale of dietary supplements defines a dietary supplement as a product other than tobacco intended to supplement the diet that bears or contains one of more of the following dietary ingredients. This included clearly labeling the product as a supplement that is meant to be ingested while differentiating the product from actual foods. Cosmetic act fd c act.